Our research aims to harness genomics and the characterization of evolutionary processes to better understand the ecology of RNA viruses across scales, from within- to between hosts, from small transmission chains to epidemics, and from deep to recent evolutionary processes. The Lequime lab focuses on RNA viruses infecting eukaryotes, from unicellular organisms to whales. While we work on a wide range of hosts, we have a special place in my heart for arthropods (mosquitoes, but not only!).
To explore these questions, we use an integrated combination of “wet” (controlled experiments, fieldwork, high-throughput sequencing) and “dry” (bioinformatics, phylodynamics, modeling, and simulations) approaches.
Constraints on viral evolution within their hosts
Because of their high mutation rate, within their hosts, viruses exist as a population of variants. This "swarm" is shaped by important constraints from their 'micro'-environment. This includes the host's genetic background, immune responses, drugs, vaccines, and tissue-specific replication dynamics. In addition, random effects, such as bottlenecks between tissues or during transmission, also significantly impact viral evolutionary dynamics.
We aim to disentangle these different constraints and characterize how they act on virus evolution. In particular, we are interested how other viruses that co-infect the host may influence evolutionary trajectories.
- Mongelli et al. (2022) Innate immune pathways act synergistically to constrain RNA virus evolution in Drosophila melanogaster. Nature Ecology & Evolution 6: 565-578. doi: 10.1038/s41559-022-01697-z
- Lequime et al. (2016) Genetic Drift, Purifying Selection and Vector Genotype Shape Dengue Virus Intra-host Genetic Diversity in Mosquitoes PLoS Genetics 12(6):e1006111 doi: 10.1371/journal.pgen.1006111
Endogenous viral elements
Endogenous viral elements (EVEs) are full or partial integrations of viral genetic material in their hosts' genomes. These integrations sometimes bring new functions, but they are a clue of the ancient history of viruses, i.e., "genomic fossils". We are interested in all aspects of RNA virus-derived EVEs, especially the role they play in virus-host interaction, on viral evolution, and what they tell us on the long and ancient evolutionary history between the viruses and their hosts.
- Brait, Hackl & Lequime (2025) detectEVE: Fast, Sensitive and Precise Detection of Endogenous Viral Elements in Genomic Data. Molecular Ecology Resources e14083 doi: 10.1111/1755-0998.14083
- Brait et al. (2024) A tale of caution: How endogenous viral elements affect virus discovery in transcriptomic data. Virus Evolution 10(1): vead088 doi: 10.1093/ve/vead088
- Li et al. (2022) Endogenous viral elements in shrew genomes provide insights into Pestivirus ancient history. Molecular Biology and Evolution 39(10): msac190 doi: 10.1093/molbev/msac190
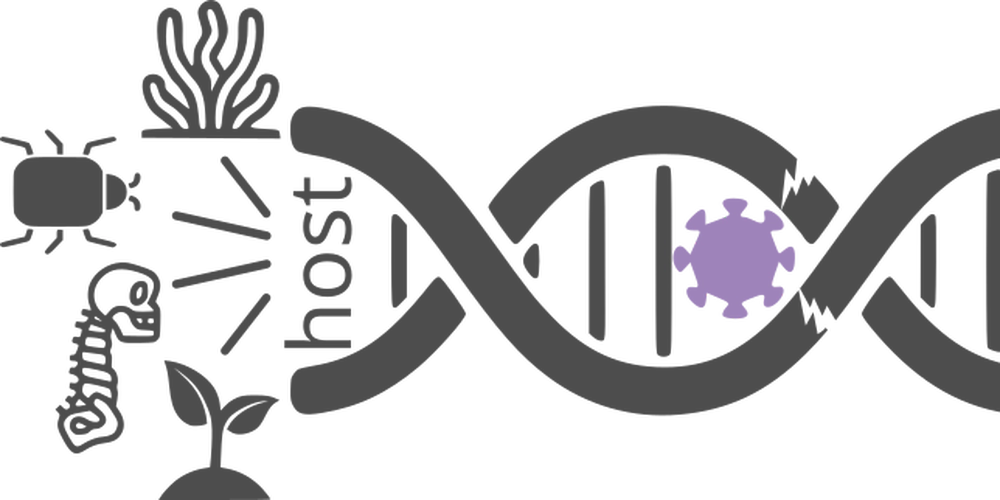
Ancient virus evolution
Archival viral genomes—recovered from museum specimens, frozen samples, and other preserved materials—are crucial for understanding the long-term evolution of viruses. While modern genomic surveillance has expanded rapidly since the 2000s, older genomes remain underrepresented in databases. These historical sequences provide essential context for identifying conserved regions, past adaptive mutations, and long-term selection pressures. Additionally, phylogenetic and molecular clock analyses using archival data help trace the origins, transmission routes, and historical dynamics of major outbreaks, offering key insights for future epidemic preparedness.
- Patrono et al. (2022) Archival influenza virus genomes from Europe reveal genomic variability during the 1918 pandemic. Nature Communications 13(1): 2314 doi: 10.1038/s41467-022-29614-9
- Düx°, Lequime° et al. (2020) Measles virus and rinderpest virus divergence dated to the sixth century BCE. Science 368(6497):1367-1370doi: 10.1126/science.aba9411
Factors influencing viral transmission
With tiny viruses, it is sometimes easy to lose the big picture. Here we aim to connect insights collaborators and we get through experimental and field-derived work into bigger scales simulation models, using our framework nosoi for the epidemiology and evolution of viruses. Once the model is set, we can use them to explore what can happen under a various set of scenarios and potentially interacting factors. For example, how would this intervention measure change the dynamics of any epidemics? How would aggressively lower the density of vector affect the spread of a vector-borne pathogen?
- Lequime et al. (2020) Modeling intra-mosquito dynamics of Zika virus and its dose-dependence confirms the low epidemic potential of Aedes albopictus. PLoS Pathogens 16(12):e1009068 doi: 10.1371/journal.ppat.1009068
- Lequime et al. (2020) nosoi: A stochastic agent-based transmission chain simulation framework in R. Methods in Ecology and Evolution 11:1002-1007 doi: 10.1111/2041-210X.13422
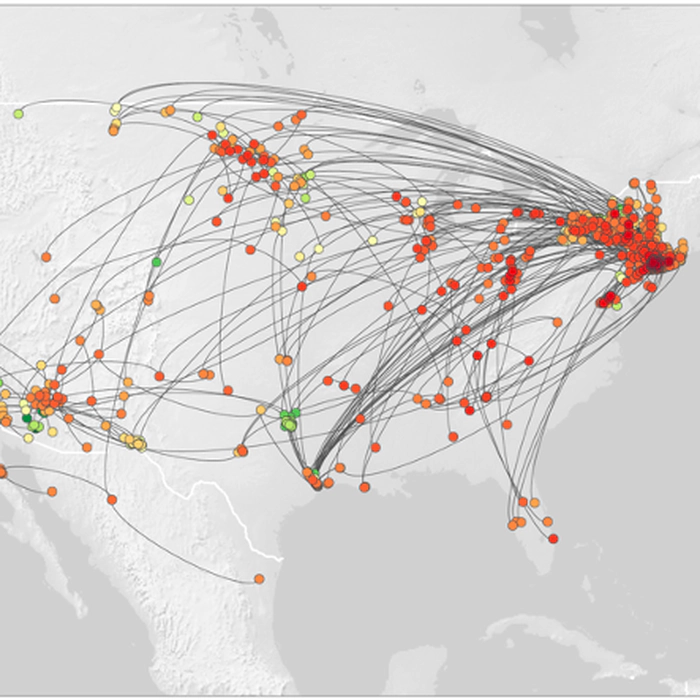
Viral ecology using genomics
Because RNA viruses evolve so quickly, their evolution occurs at the same timescale as their ecological (or epidemiological) processes. By reconstructing the evolutionary of a set of viral genomic sequences, we can infer some insights into their ecology, e.g. how do they spread, and where from? What are the factors that favor the spread?
- Dellicour et al. (2025) Comparative performance of novel viral landscape phylogeography approaches. PNAS 122(26):e2506743122 doi:10.1073/pnas.2506743122
- Dellicour et al. (2020) Epidemiological hypothesis testing using a phylogeographic and phylodynamic framework. Nature Communications 11(1):5620 doi: 10.1038/s41467-020-19122-z